Memo #315
By Yifan Wang – yfwang [at] essex.ac.uk
 Drug regulations in China stipulate that chemical and generic names of drugs are determined by the Chinese Pharmacopeia (Ch.P) and the State Food and Drug Administration (SFDA), while brand names are chosen by pharmaceutical companies, as long as they are recorded with the SFDA. Some Chinese pharmaceutical companies take advantage of this policy loophole that allows them to name their own drugs by marketing their products as “unique” and “superior” in the expectation of generating greater profits. This marketing technique to differentiate their product from existing drugs has the potential to mislead consumers into believing a “new” type of drug has an improved therapeutic effect. Thus, some pharmaceutical companies regularly change the brand name for an existing product, or add multiple dosages/name combinations, rather than pursuing real innovation. Obviously, this government drug regulation leaves plenty of room for manufacturers to circumvent government price regulations. Introducing a new drug is a means by which the industry can justify a higher price. However, in China as in the United States, it has been shown that “most new drugs offer little or no advantage over existing drugs to offset their greater risk.”
Drug regulations in China stipulate that chemical and generic names of drugs are determined by the Chinese Pharmacopeia (Ch.P) and the State Food and Drug Administration (SFDA), while brand names are chosen by pharmaceutical companies, as long as they are recorded with the SFDA. Some Chinese pharmaceutical companies take advantage of this policy loophole that allows them to name their own drugs by marketing their products as “unique” and “superior” in the expectation of generating greater profits. This marketing technique to differentiate their product from existing drugs has the potential to mislead consumers into believing a “new” type of drug has an improved therapeutic effect. Thus, some pharmaceutical companies regularly change the brand name for an existing product, or add multiple dosages/name combinations, rather than pursuing real innovation. Obviously, this government drug regulation leaves plenty of room for manufacturers to circumvent government price regulations. Introducing a new drug is a means by which the industry can justify a higher price. However, in China as in the United States, it has been shown that “most new drugs offer little or no advantage over existing drugs to offset their greater risk.”
As a result of all this, in recent years, China has seen an increasing number of new brand names for the same drug, or the appearance of the same product but with new dosages or package units, along with increasingly aggressive marketing campaigns. According to official statistical data, in China among the 200 types of commonly used drugs, those with at least 4 brand names account for 20%, while some 80% of these have four or more brand names. The phenomenon of “one drug with multiple names” is increasingly prevalent, especially for those drugs widely used in clinical practice, such as antibiotics and cardiovascular medications.
In order to reduce this superficial differentiation of “new” drugs and to develop real R&D, the Chinese government will need to focus on policy design, implementation, supervision and evaluation for the nation’s pharmaceutical industry. This issue also sheds critical light on the current reforms addressing, and the effective interaction of, the state and the pharmaceutical industry in China’s healthcare domain.
About the Author:
Yifan Wang is a PhD candidate in the Department of Sociology, University of Essex.

In one Shandong pharmacy, drugs with the same ingredients are being sold under various names and with different dosages or units (Credit: Yifan Wang).
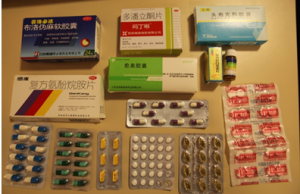
On the packages of their medications, Chinese pharmaceutical manufactures make the generic name less visible to consumers (Credit: Yifan Wang).
Links:
- Donald W. Light, The Risk of Prescription Drugs (New York: Columbia University Press), 2010
- “‘One drug with multiple names’: the underlying profit chain,” Guangming Online, March 2006 (in Chinese)
- Zhang Wei Wilson & X. Liu, “Challenges to China’s Pharmaceutical Industry and Their Policy Implication,” in Karen Eggleston (ed.), Prescribing Cultures and Pharmaceutical Policy in the Asia-Pacific (Walter H. Shorenstein Asia-Pacific Research Center), 2009
- Q. Sun & Q. Meng, “Pharmaceutical Policy and the Appropriate Use of Medicines in China,” in Karen Eggleston (ed.), Prescribing Cultures and Pharmaceutical Policy in the Asia-Pacific (Walter H. Shorenstein Asia-Pacific Research Center), 2009
- “‘One drug many names’ is cheating the public,” Xinhua News, September 2007 (in Chinese)
Related Memos:
See our other memos on China.
Comments are closed, but trackbacks and pingbacks are open.